Patients who have been prescribed PAXLOVID may be eligible for support resources through PAXCESS so they can get their prescription as soon as possible.
Patients with commercial insurance can pay as little as $0* for PAXLOVID.
Patients who are commercially insured, meaning they have private health insurance usually through an employer, may be eligible to save with the PAXCESS Co-Pay Savings Program. Here's how:
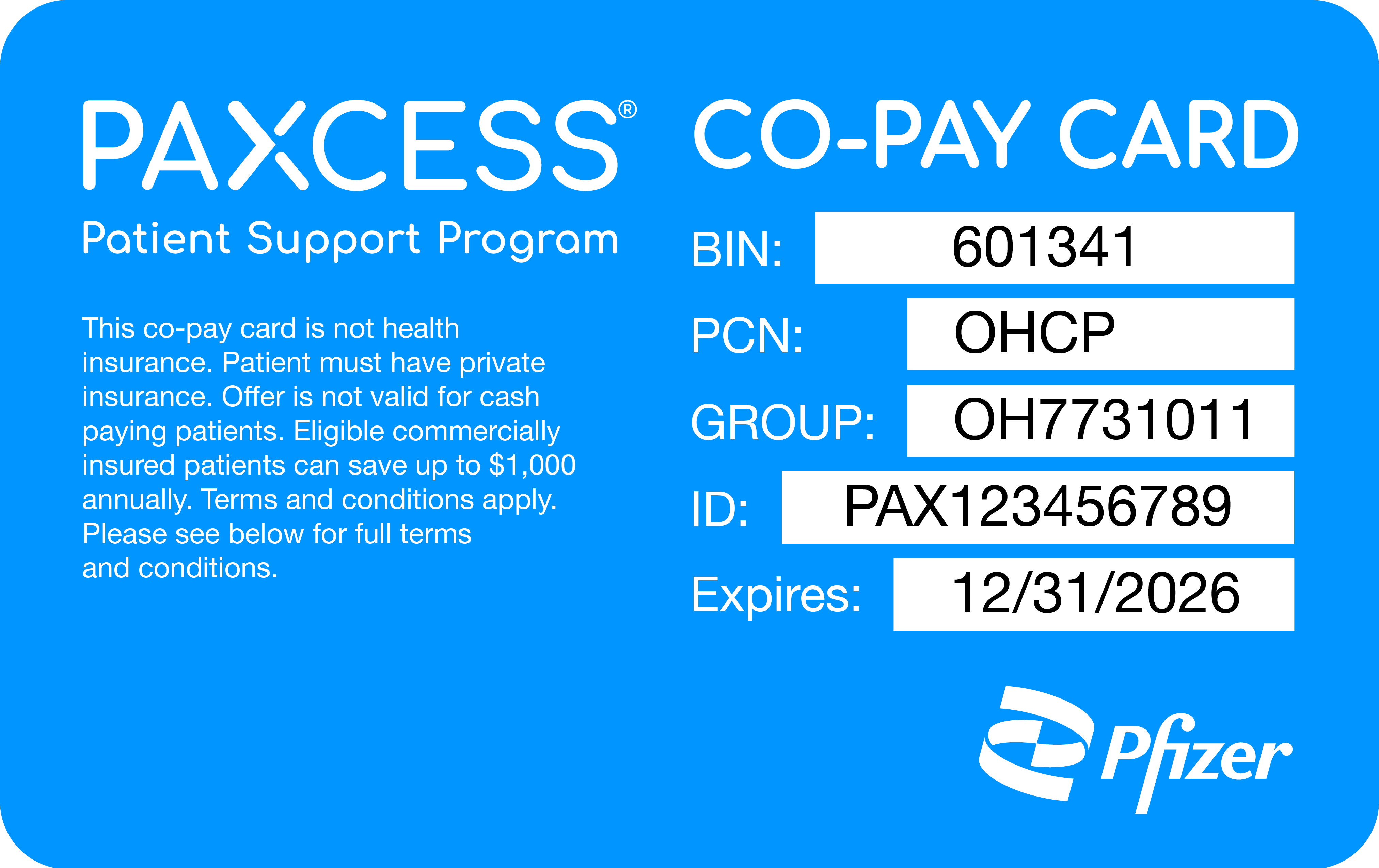
*Eligible commercially insured patients can save up to a maximum of $1,000 annually. Terms and conditions apply.
PAXLOVID is authorized for
emergency use
- PAXLOVID has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in pediatric patients 12 years of age and older weighing at least 88 pounds (40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death.
- The emergency use of PAXLOVID is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner.

The U.S. Government Patient Assistance Program (USG PAP) is operated by Pfizer to help eligible patients without commercial insurance get PAXLOVID for free.
To enroll in the USG PAP, call 1-877-219-7225 or visit the PAXCESS Patient Portal.

on this site are actors.
Patients may be able to get their prescription at their local pharmacy or via overnight mail order.‡ To find a pharmacy participating in the USG PAP or to arrange overnight shipping, call 1-877-219-7225.
Patients may be able to get their prescription at their local pharmacy or via overnight mail order.‡ To find a pharmacy participating in the USG PAP or to arrange overnight shipping, call
PAXCESS offers personalized
resources to help people get
their
prescription.
PAXCESS helps eligible patients save on their prescription costs and get their PAXLOVID as soon as possible with resources like:
Insurance verification
Help with identifying financial assistance
Support with program enrollment, if eligible
Live PAXCESS representatives who can help patients understand their insurance benefits and program eligibility
Enrollment in the PAXCESS Patient Support Program can be completed by the patient or caregiver. Enrollment can take approximately 5 minutes.§
†Eligible government insured and uninsured patients can access PAXLOVID for free through December 31, 2026. The USG PAP operated by Pfizer is an independent program with separate eligibility requirements offered by the United States Department of Health and Human Services and is not owned by Pfizer. Terms and conditions apply.
‡Exceptions include but may not be limited to: cutoff for overnight orders Monday-Friday 3 PM ET; no delivery on Sunday; Saturday delivery available in select metropolitan areas.
§Actual times may vary.

Find a pharmacy participating in the USG PAP
See pharmacy finder

